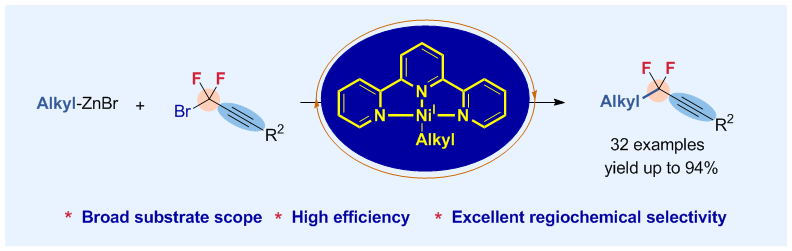
In spite of the important applications of difluoroalkylated molecules in medicinal chemistry, to date, the reaction of difluoroalkylating reagents with unactivated, aliphatic substrates through a controllable manner remains challenging and has not been reported. Here we describe an efficient nickel-catalyzed cross-coupling of unactivated alkylzinc reagen\ts with gemdifluoropropargyl bromides. The reaction proceeds under mild reaction conditions with high efficiency and excellent regiochemical selectivity. Transformations of the resulting difluoroalkylated alkanes lead to a variety of biologically active molecules, providing a facile route for applications in drug discovery and development.
More details: https://www.nature.com/articles/s41467-017-01540-1
|