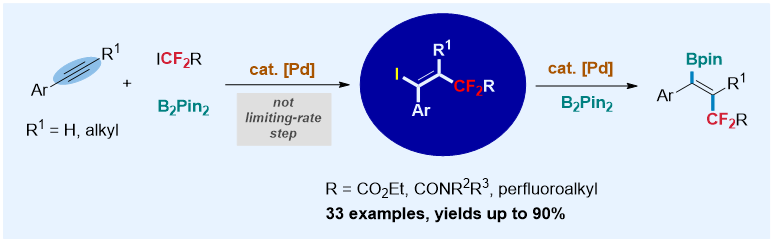
Herein, we report a palladium-catalyzed trans-fluoroalkylation-borylation of alkynes with fluoroalkyl iodides and B2pin2. The reaction tolerates a series of difluoroalkyl iodides and perfluoroalkyl iodides and can enable coupling with a variety of alkynes, including internal and terminal alkynes, with high efficiency, high functional group compatibility, and high regio- and stereoselectivities. Preliminary mechanistic studies reveal that a transfluoroalkylated alkenyl iodide is the key intermediate, which subsequently undergoes borylation to produce the transfluoroalkylated alkenylboronate.
More Details: https://pubs.acs.org.ccindex.cn/doi/10.1021/acscatal.8b02842
|