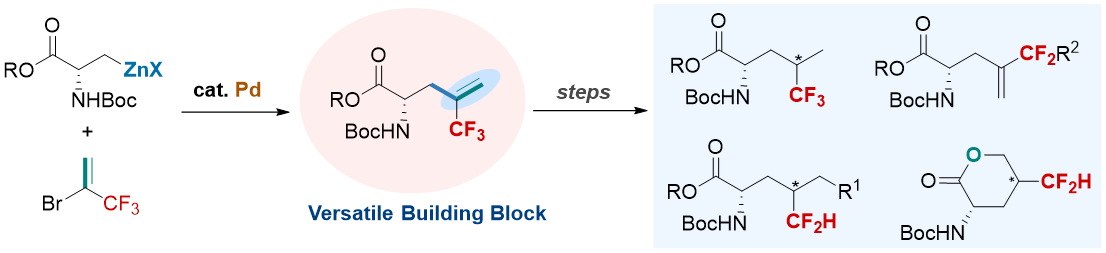
A palladium-catalyzed cross-coupling of unactivated alkylzinc reagents with 2-bromo-3,3,3-trifluoropropene (BTP) has been developed, which was used as a key step to prepare a series of trifluoromethylated and difluoromethylated amino acids that are of great interest in peptide/ protein based chemical biology. The advantages of the synthesis of these fluorinated amino acids are synthetic simplicity and diversity from a simple and readily available key intermediate a-trifluoromethylalkenecontaining amino acid, providing a facile route for applications in medicinal chemistry and life science.
More Details: https://pubs.rsc.org/en/Content/ArticleLanding/2019/CC/C8CC10212K#!divAbstract
|