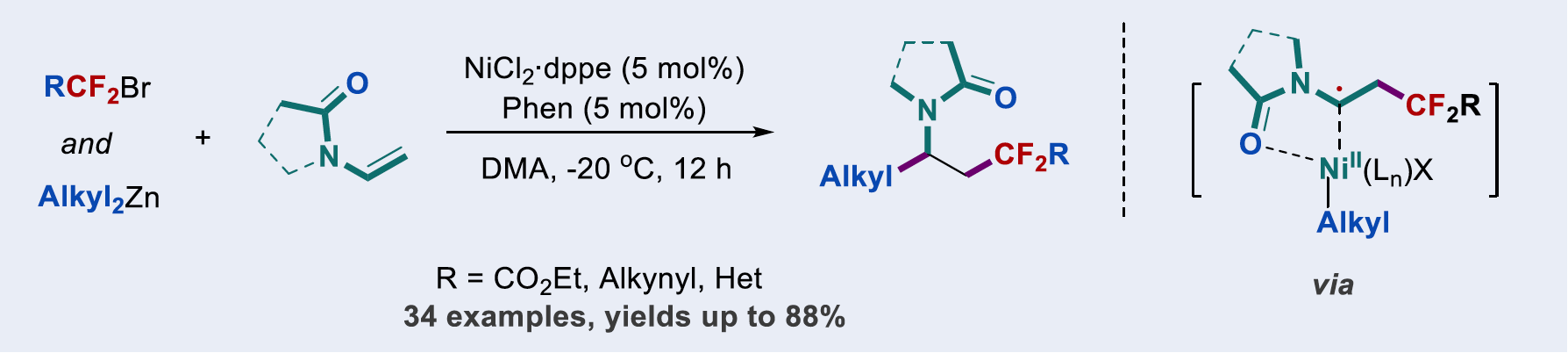
Nickel-catalyzed carbodifunctionalization of alkenes is an efficient strategy for the construction of C–C bonds. However, applications of the strategy in dialkylation of alkenes remain underdeveloped due to the difficulties in suppressing competitive side reactions. We now describe a nickel-catalyzed tandem reaction by difluoroalkylation–alkylation of N-vinyl 2-pyrrolidinone with difluoroalkyl bromides and dialkylzinc reagents. The reaction can also extend to N-vinyloxazolidinone and N-vinylacetamide. This carbodifunctionalization reaction proceeds smoothly under mild reaction conditions with good functional group tolerance, providing a straightforward access to gem-difluoroalkylated 2-pyrrolidinone derivatives that are of interest in medicinal chemistry.
More Details: https://pubs.acs.org/doi/10.1021/acscatal.9b02488
|