Difluorocarbene has important applications in pharmaceuticals, agrochemicals and materials, but all these applications proceed using just a few types of reaction by taking advantage of its intrinsic electrophilicity. Here, we report a palladium-catalysed strategy that confers the formed palladium difluorocarbene(Pd=CF2 )species with both nucleophilicity and electrophilicity by switching the valence state of the palladium centre (Pd(0) and Pd(II), respectively). From just this simple fluorine source BrCF2PO(OEt)2 , difluorocarbene transfer enables access to four types of product: difluoromethylated and tetrafluoroethylated arenes and their corresponding fluoroalkylated ketones.
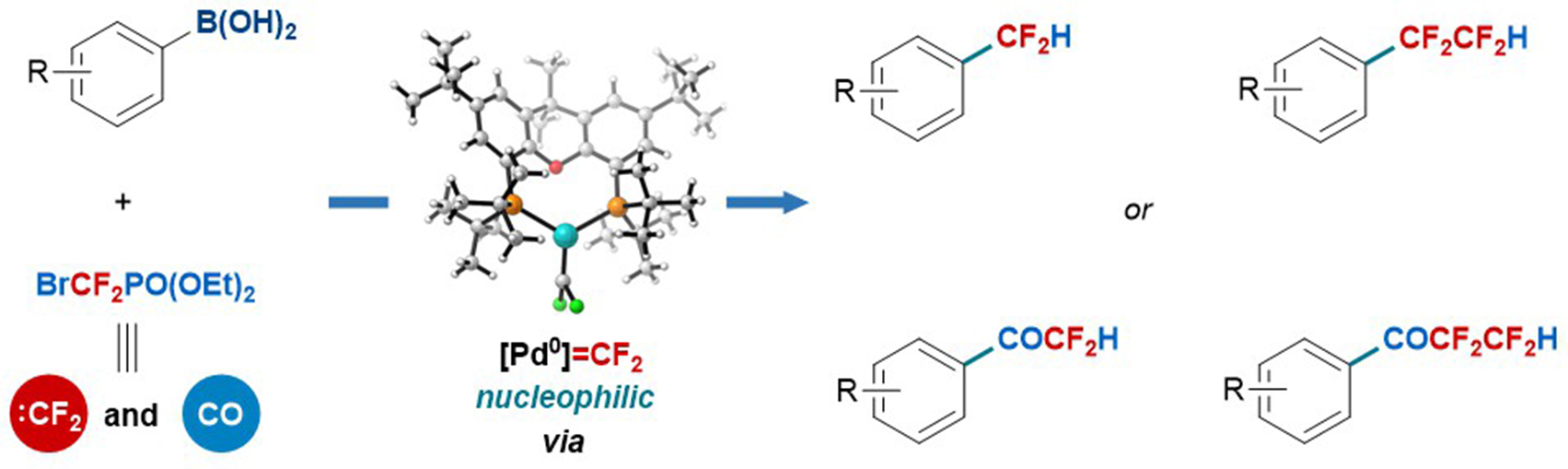
For detail:https://www.nature.com/articles/s41557-019-0331-9
|